Details
-
Type:
Task
-
Status: Closed (View Workflow)
-
Priority:
Major
-
Resolution: Done
-
Affects Version/s: None
-
Fix Version/s: None
-
Labels:None
-
Story Points:5
-
Epic Link:
-
Sprint:Spring 3, Spring 4, Spring 5, Spring 6, Spring 7
Description
Data has been submitted to SRA and now needs to be reviewed. To do this we perform a rerun of the data by pulling it from SRA directly and pushing it through the nextflow pipeline and prepping it for IGB quick load. To confirm that the data on SRA is correct we can make a few comparisons with the original data.
- Compare files sizes of the original data to the new rerun file sizes.
- Open IGB and compare bam and coverage graphs with the original and rerun data.
- Perform these comparisons for SL4 and SL5
Muday cluster Directories:
Original: /projects/tomato_genome/fnb/dataprocessing/muday-144
Re-run: /projects/tomato_genome/fnb/dataprocessing/SRP460750
Attachments
Issue Links
- relates to
-
IGBF-3627 Create sample sheet for SRP460750
-
- Closed
-
-
IGBF-3639 Compare original mark-2022-timeseries data to SRA Re-run data
-
- Closed
-
-
IGBF-3683 Update SRA to use the correct sample codes for Muday lab time course data
-
- Closed
-
-
IGBF-3346 Prepping data and entries for SRA for Muday time course
-
- To-Do
-
-
IGBF-3290 Use new sample labels recommended by Muday Lab
-
- Closed
-




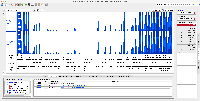
Download Data from Cluster:
Original Muday Bam files:
Rerun Muday Bam files:
Original Muday Scaled Coverage Graphs:
Rerun Muday Scaled Coverage Graphs:
Notes: Had to compress bam files in each directory before downloading. I also have to use 'Muday-lab-RNA-samples-for-sample-name-conversion' file from flavonoid repo to check the correct names of the samples due to mistakes in the lab which led to sample switching.